Facebook will soon label all posts discussing the coronavirus vaccination with a pointer to official information about COVID-19, it said today.
It also revealed it has implemented some new “temporary” measures aimed at limiting the spread of vaccine misinformation/combating vaccine hesitancy — saying it’s reducing the distribution of content from users that have violated its policies on COVID-19 and vaccine misinformation; or “that have repeatedly shared content debunked as False or Altered by our third-party fact-checking partners”.
It’s also reducing distribution of any COVID-19 or vaccine content that fact-checking partners have rated as “Missing Context”, per the blog post.
While admins for groups with admins or members who have violated its COVID-19 policies will also be required to temporarily approve all posts within their group, it said. (It’s not clear what happens if a group only has one admin and they have violated its policies.)
Facebook will also “further elevate information from authoritative sources when people seek information about COVID-19 or vaccines”, it added.
It’s not clear why users who repeatedly violate Facebook’s COVID-19 policies do not face at least a period of suspension. (We’ve asked the company for clarity on its policies.)
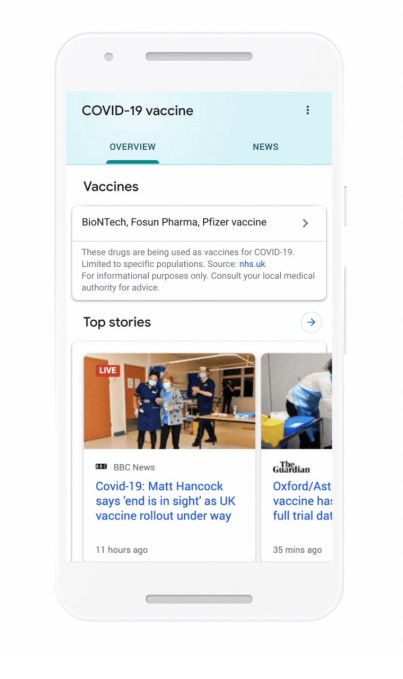
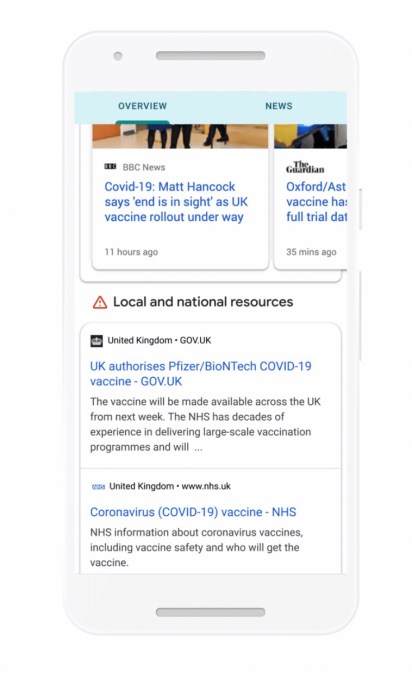
“We’re continuing to expand our efforts to address COVID-19 vaccine misinformation by adding labels to Facebook and Instagram posts that discuss the vaccines,” Facebook said in the Newsroom post today.
“These labels contain credible information about the safety of COVID-19 vaccines from the World Health Organization. For example, we’re adding a label on posts that discuss the safety of COVID-19 vaccines that notes COVID-19 vaccines go through tests for safety and effectiveness before they’re approved.”
The incoming COVID-19 information labels are rolling out globally in English, Spanish, Indonesian, Portuguese, Arabic and French (with additional languages touted “in the coming weeks”), per Facebook.
As well as soon rolling out labels “on all posts generally about COVID-19 vaccines” — pointing users to its COVID-19 Information Center — Facebook said it would add additional “targeted” labels about “COVID-19 vaccine subtopics”. So it sounds like it may respond directly to specific anti-vaxxer misinformation it’s seeing spreading on its platform.
“We will also add an additional screen when someone goes to share a post on Facebook and Instagram with an informational COVID-19 vaccine label. It will provide more information so people have the context they need to make informed decisions about what to share,” Facebook added.
The moves follow revelations that an internal Facebook study of vaccine hesitancy — which was reported on by the Washington Post yesterday after it obtained documents on the large-scale internal research effort — found a small number of US users are responsible for driving most of the content that’s hesitant about getting vaccinated.
“Just 10 out of the 638 population segments [Facebook’s study divided US users into] contained 50 percent of all vaccine hesitancy content on the platform,” the Post reported. “And in the population segment with the most vaccine hesitancy, just 111 users contributed half of all vaccine hesitant content.”
Last week the MIT Technology Review also published a deep-dive article probing Facebook’s approach to interrogating, via an internal ‘Responsible AI’ team, the impacts of AI-fuelled content distribution — which accused the company of prioritizing growth and engagement and neglecting the issue of toxic misinformation (and the individual and societal harms that can flow from algorithmic content choices which amplify lies and hate speech).
In the case of COVID-19, lies being spread about vaccination safety or efficacy present a clear and present danger to public health. And Facebook’s PR machine does appear to have, tardily, recognized the extent of the reputational risk it’s facing if it’s platform is associated with driving vaccine hesitancy.
To wit: Also today it’s announced the launch of a global COVID-19 education drive that it says it hopes will bring 50M people “closer to getting vaccinated”.
“By working closely with national and global health authorities and using our scale to reach people quickly, we’re doing our part to help people get credible information, get vaccinated and come back together safely,” Facebook writes in the Newsroom post that links directly to a Facebook post by founder Mark Zuckerberg also trailing the new measures, including the launch of a tool that will show U.S. Facebook users where they can get vaccinated and provide them with a link to make an appointment.
Facebook said it plans to expand the tool to other countries as global vaccine availability steps up.
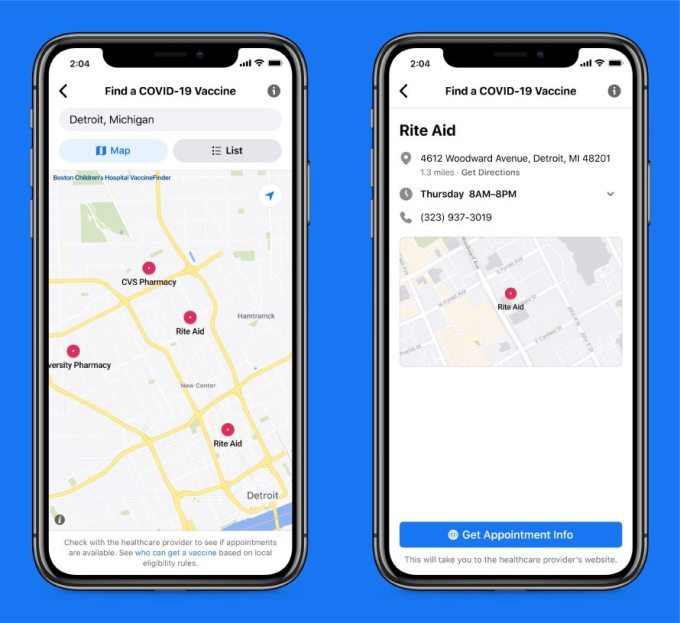
Facebook’s vaccine appointment finder tool (Image credits: Facebook)
Facebook has further announced that the COVID-19 information portal it launched in the Facebook app in March last year which points users to “the latest information about the virus from local health ministries and the World Health Organization” is finally being brought to Instagram.
It’s not clear why Facebook hadn’t already launched the portal on Instagram. But it’s decided to double down on fighting bad speech (related to vaccines) with better speech — saying Instagram users will get new stickers they can add to their Instagram Stories “so people can inspire others to get vaccinated when it becomes available to them”.
In other moves being trailed in Facebook’s crisis PR blitz today it has touted “new data and insights” on vaccine attitudes being made available to public officials via COVID-19 dashboards and maps it was already offering (the data is collected by Facebook’s Data for Good partners for the effort at Carnegie Mellon University and University of Maryland as part of the COVID-19 Symptom Survey).
Albeit, it doesn’t specify what new information is being added there (or why now).
Also today it said it’s “making it easy to track how COVID-19 vaccine information is being spread on social media through CrowdTangle’s COVID-19 Live Displays“.
“Publishers, global aid organizations, journalists and others can access real-time, global streams of vaccine-related posts on Facebook, Instagram and Reddit in 34 languages. CrowdTangle also offers Live Displays for 104 countries and all 50 states in the US to help aid organizations and journalists track posts and trends at a regional level as well,” Facebook added, again without offering any context on why it hadn’t made it easier to use this tool to track vaccine information spread before.
Its blog post also touts “new” partnerships with health authorities and governments on vaccine registration — while trumpeting the ~3BN messages it says have already been sent “by governments, nonprofits and international organizations to citizens through official WhatsApp chatbots on COVID-19”. (As WhatsApp is end-to-end encrypted there is no simple way to quantify how many vaccine misinformation messages have been sent via the same platform.)
Per Facebook, it’s now “working directly with health authorities and governments to get people registered for vaccinations” (such as in the city and province of Buenos Aires, Argentina, which is using WhatsApp as the official channel to send notifications to citizens when it’s their turn to receive the vaccine).
“Since the beginning of the COVID-19 pandemic, we have partnered with ministries of health and health-focused organizations in more than 170 countries by providing free ads, enabling partners to share their own public health guidance on COVID-19 and information about the COVID-19 vaccine,” Facebook’s PR adds in a section of the post which it’s titled “amplifying credible health information and resources from experts”.